
For superficial, deep cornea ulcers and/or infections in small animals;
150~300microns, in anterior superficial lamellar keratoplasty for non-penetrating cases;
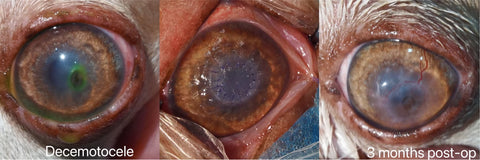
350~400microns, in posterior deep lamellar keratoplasty for Descemetocele cases;
450-500microns, in penetrating cases;
550-600microns, for horse or large animals.
Diameters: 10/12mm.
Storage and Transportation:
Room temperature, in refrigerator (2-8 degrees Celsius)is better.
Shelf Life: 18 months.
One unit per one box.
PetsEyes BioCorneaVet™ is a kind of Decellularized Porcine Corneal Xenografts, specifically developed and designed in the use of veterinary ophthalmology. This graft preserves the complex features that are essential to corneal function, including light transparency, resistance to ultraviolet light, insensitivity to photons and good tensile strength. And the decellularized methods used in the preparation of the graft maximize the elimination of cellular components and nuclear materials, while preserving the extracellular matrix(ECM) proteins and mechanical properties. The porous scaffold structure is an ideal platform for the ingrowth of nerve and vessels as well as the repopulation of host cells like keratocytes and epithelial cells.
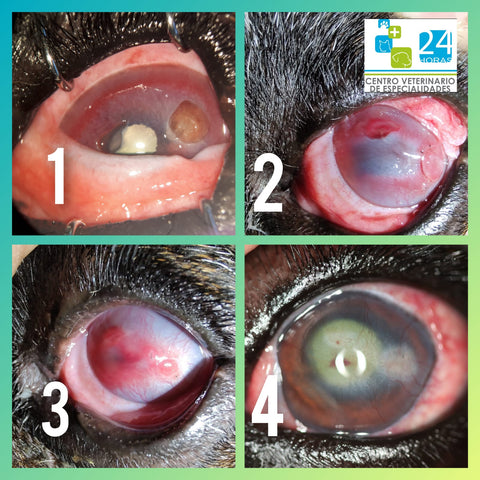
This graft is indicated for the treatment of corneal diseases, ulcerations, descemetocele, perforations, feline corneal sequestrum, and trauma where pharmaceutical interventions and/or temporary ocular bandage coverage have failed or are of no use. But the professional veterinary ophthalmologists are the final decision-maker in using this graft or other methods to repair the corneal defects.
In order to successfully use this graft in veterinary ophthalmology, there are a handful of key points needed to be paid attentions.
a. The rehydration time shall be strictly limited within 10-20 seconds, no longer than 60 seconds. And avoid frequent flushing during the suture to prevent the graft from the over-edema.
b. Gently suture the graft and avoid any extra stretch of the stitches on the graft. Intermittent suturing is preferred. 9-0 or 10-0 (absorbable or non-absorbable) stitches shall be applied. The graft size should be 0.25-1.0 mm larger than the recipient's corneal dissection size.
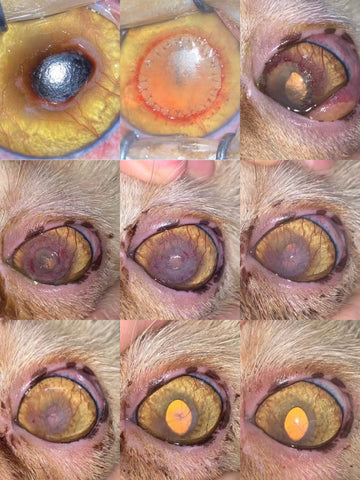
c. The unfulfilled re-epithelialization is the major reason for the dissolution of all grafting source (included but not limited autologous, homogeneous and heterogenous) and the failure of the keratoplasty. Therefore, the re-epithelialization time that would be normally completed in 3-10 days, no longer than 14 days shall be closely observed that is the most key factor for the survival of the graft after the transplantation.
The post-op use of contact lens is also recommended to promote the completion of re-epithelialization.
Under the normal conditions(without infections and/or the effects from MMPs-matrix metalloproteinases), the graft can be endurable as long as 1 month without the protection of epithelial layer.
When the re-epithelialization cannot be fulfilled in 14 days and longer than 21 days, the active methods to maintain the integrity of epithelial layer shall be applied, like the grafting of amniotic membrane.
d. The major reasons for the unfulfilled re-epithelialization include reinfections, the in-compliance of eye drops, rejection, deficiency of limbus stem cells, dry eye and etc.
The eye drops of anti-microorganisms are recommended before and after the keratoplasty in order to treat the existent infections and prevent potential infections or reinfections that are one of the major reasons for the graft dissolution. The active phase of viral infection is predisposed for the dissolution of the graft.
The eye drops of and artificial tears or similars, bovine serum, autologous serum and growth factors are highly recommended to promote the completion of re-epithelialization.
For the BioCorneaVet, the graft dissolution caused by rejection from the xenogeneic source is seldomly observed. The neovascularity of the graft after the surgery is generally regarded as a normal part of granulation in the process of wound healing.
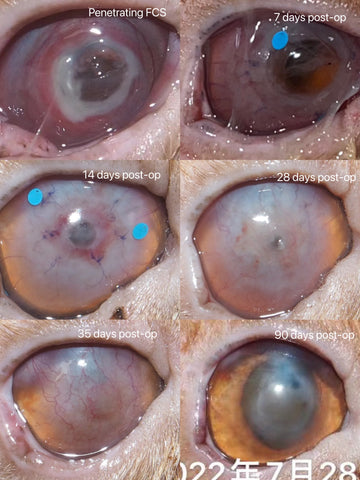
e. The first 14 days after the keratoplasty is very crucial for the final success of the transplantation.
For penetrating keratoplasty, the graft on the hole area may be seen as thinning, de-compensating or penetrating in the first 14 days, but it is still there and just wait the re-epithelialization and the bio-integration of the graft to be fully completed after 14 days. The leakage of aqueous humor and/or increased secretions in the first 7 days are regarded as normal signs.
f. The full healing duration for lamellar keratoplasty is approximately 4-8 weeks, and 12-14 weeks for deep lamellar or penetrating keratoplasty.
g. The graft would be gradually re-modeling a portion of the recipient’s cornea and gradually restore its original thickness with the recession of the newly developed hyper-vascular tissues that commonly occurred from the 4th week post-operatively.
h. The fully restoration of the recipient’s corneal transparency would be achieved for lamellar keratoplasty like most feline sequestrum and those with descemetocele that is the most significant value for this graft, while the preservation of the recipient’s eyeball and some extend cosmetic and vision functions would be realized for those who are suffered from fully penetration injuries and administered penetrating keratoplasty.

1. Lavaud A, Kowalska ME, Voelter K, Pot SA, Rampazzo A. Penetrating Keratoplasty in Dogs using Acellular Porcine Corneal Stroma (BioCorneaVetTM): A prospective pilot study of five cases. Vet Ophthalmol. 2021;00:1–11. https:// doi.org/10.1111/vop.12884
2. Santillo D, Mathieson I, Corsi F, Göllner R, Guandalini A. The use of acellular porcine corneal stroma xenograft (BioCorneaVetTM) for the treatment of deep stromal and full thickness corneal defects: A retrospective study of 40 cases (2019–2021). Vet Ophthalmol. 2021;00:1–15. https://doi.org/10.1111/vop.12927
3. Xu, H.; Sapienza, J.S.; Jin, Y.; Lin, J.; Zheng, X.; Dong, H.; Diao, H.; Zhao, Y.; Gao, J.; Tang, J.; et al. Lamellar Keratoplasty Using Acellular Bioengineering Cornea (BioCorneaVetTM) for the Treatment of Feline Corneal Sequestrum: A Retrospective Study of 62 Eyes (2018–2021). Animals 2022, 12, 1016. https://doi.org/10.3390/ani12081016















